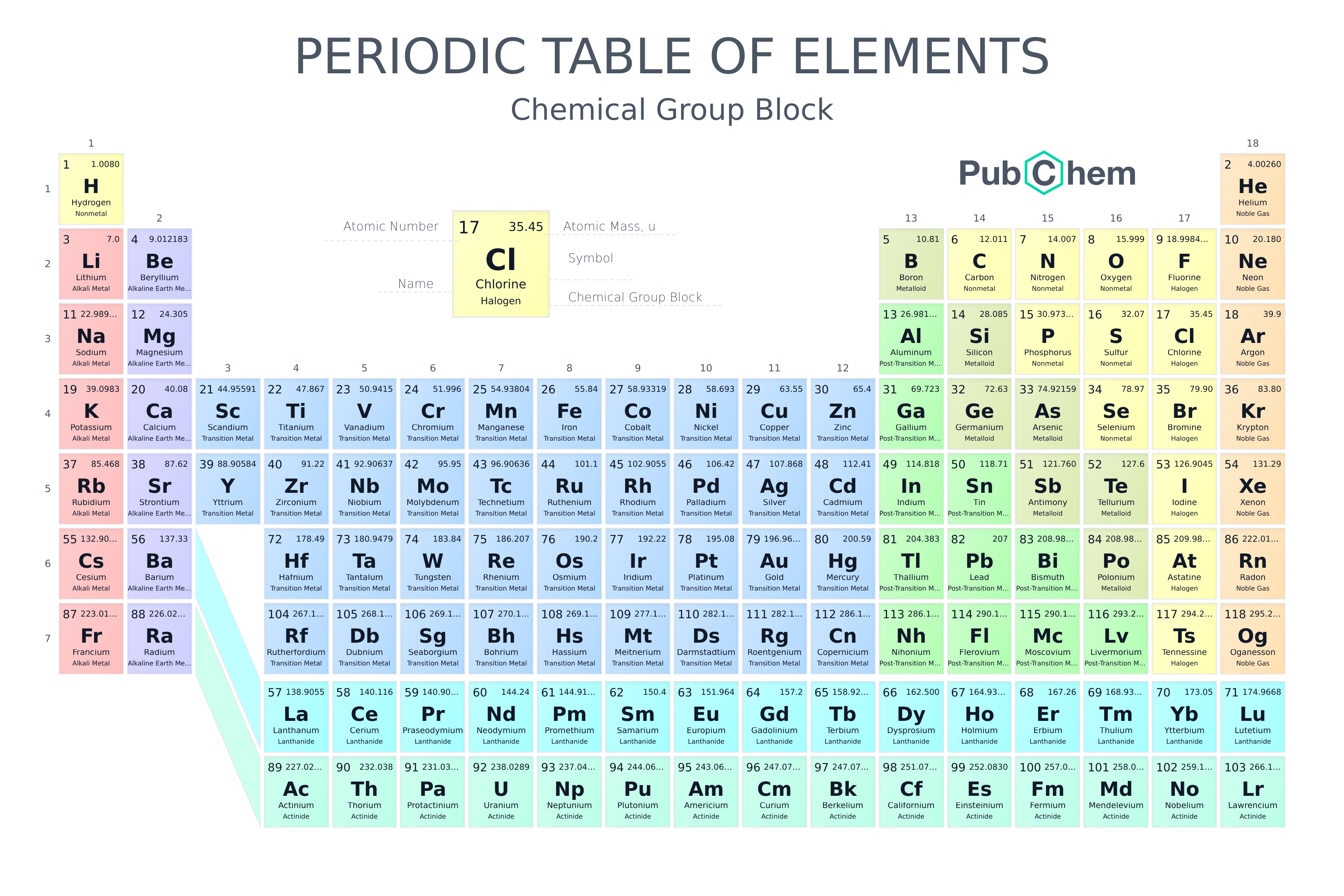
If you know little about chemistry then by now you know that the world of chemistry is infinite. In 1800 only 31 elements were known, in 1865 scientists were able to identify 63 elements, right now we have 118 elements and in future, we will be able to know the existence of other elements too.
Elements with a higher atomic number were not found natural but were synthesized in laboratories like Og, Lv, Ts, Mc, etc. We have all types of elements with similar properties, different properties, with intermediate properties and different variations of chemical reactions. So to make things easy we created the periodic table, now called a modern periodic table to understand how the chemistry of this universe works and how elements(atoms) show a sequence with increasing atomic number. 原子序数较高的元素(例如Og、Lv、Ts、Mc等)并非天然元素,而是在实验室合成的。方便起见,我们创建了元素周期表,现在被称为现代元素周期表,以了解这个世界的化学原理,以及元素(原子)如何随着原子序数的增加呈现出周期性变化。
The periodic table as we know it today is managed by the International Union of Pure and Applied Chemistry, or IUPAC (eye-you-pack). 我们今天所知的元素周期表是由国际纯粹与应用化学联合会(简称IUPAC,音eye-you-pack)管理的。
While much of what is in the periodic table is stable and unlikely to change, the IUPAC organization is responsible for deciding what needs to be changed. They have created criteria for what constitutes the discovery of a new element. IUPAC负责决定需要改变的内容,他们为新元素的发现制定了标准。(虽然元素周期表中的大部分内容是固定的,不太可能改变。)
In addition, any new element must be assigned a temporary name and symbol, and if validated, given an official name. Such was the case when IUPAC recently reviewed elements 113, 115, 117 and 118, and decided to give them official names and symbols (goodbye, ununseptium and hello, tennessine!). 另外,任何新元素都必须指定一个临时名称和符号,如果经过确认,还必须指定一个正式名称。IUPAC最近审查了113号、115号、117号和118号元素,并决定给它们正式的名称和符号。
Atomic weights found within a periodic table one might think are constant. The truth is that atomic weights have changed as a function of time. Since 1899 the IUPAC Commission on Isotopic Abundances and Atomic Weights (CIAAW) has been evaluating atomic weights and abundances. For example, Carbon had an atomic weight of 12.00 in 1902 but today it is [12.0096, 12.0116]! Times sure have changed as the source of the sample will determine the value. 元素周期表中的原子量可以认为是常数。可事实上原子量随时间而变化。自1899年以来,IUPAC同位素丰度与原子量委员会(CIAAW)一直在评估原子量和丰度。例如,碳在1902年的原子量是12.00,但现在是[12.0096,12.0116]!数值由样本来源决定,因此肯定会随时间变化。
Finally, IUPAC assigns collective names (lanthanoids and actinoids) and group numbering (1 to 18) and has investigated the membership of the group 3 elements. 最后,IUPAC指定了集体名称(镧系和锕系)和族号(1至18),并研究了第3族元素。