-
Notifications
You must be signed in to change notification settings - Fork 0
Conformity Assessment and Tooling
NOTE: THIS PAGE IS STILL UNDER DEVELOPMENT!
The SES+MDI program has a key focus on realizing an ecosystem of plug-and-trust interoperable medical technology PRODUCTS. To achieve this, a robust testing and product conformity assessment (CA) must be established and maintained. Given the complexity of these technologies and the high risk - even regulatory oversight - of many of these products, additional rigor must be supported by the CA program, and a new level of implementer support (i.e., product developers) must be established.
For example, SES+MDI product developers already have to use requirements management tooling that is linked to product capabilities and standards-based requirements that are then extensively tested … all part of the V-Model for product development and existing conformity assessment programs. Thus the Gemini RI+MC+RR provides the requirements formalization + traceability & coverage from CA that is needed to better support adoption of open standards-based interoperability technology - Gemini SES+MDI!
In understanding the overall information flows and ecosystem workflow "orchestration" associated with this program, the following "quick & dirty" graphic was created:
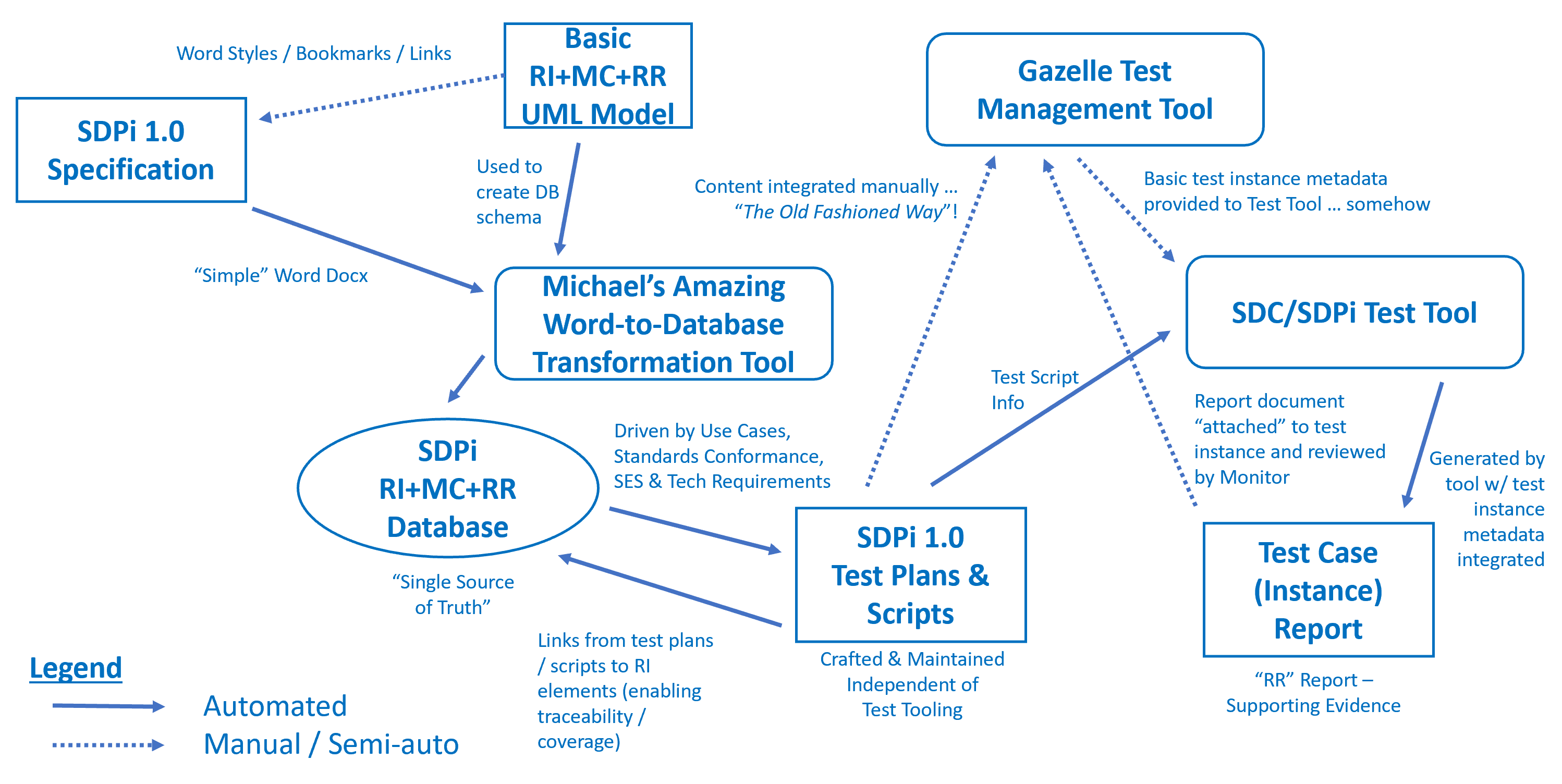
The following articles provide background rationale and guidance for a robust conformity assessment and tooling strategy in support of the Gemini SES+MDI product ecosystem …
-
CA Workflow for RI+MC+RR
-
Managing Test Assertions for RI+MC+RR
-
Test Artifacts - Formatting Integrating Creating
-
CA Testing for SES+MDI Assurance Case Evidence
-
<article introducing the EP CA guidance/strategy/plan document>
-
…
Gemini SES+MDI Program
Gemini SES+MDI Program articles …