How to use plugin (Text Tutorial)
Open the image to be analyzed in ImageJ. In general, the plugin works in two modes:
- particles detection (whole image or ROI)
- particles detection and colocalization analysis (whole image or ROI).
For colocalization you need a color composite image containing multiple color channels (two or more). Plugin auto-detects the number of channels and if it is more than one then the plugin will automatically switch to second mode (see below).
Plugin works with time/z-stacks.
Launch the detection by choosing Plugins->ComDet->Detect Particles.
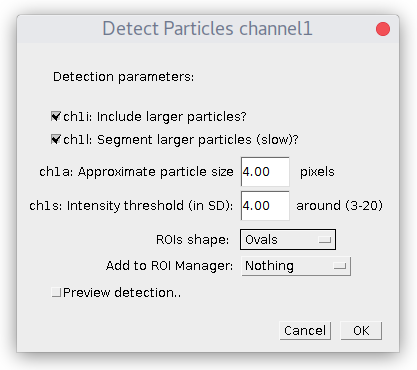
If the image is not multi-channel image, the following dialog window will appear:
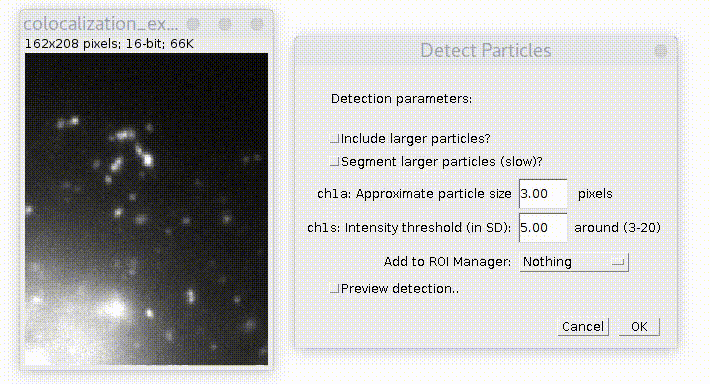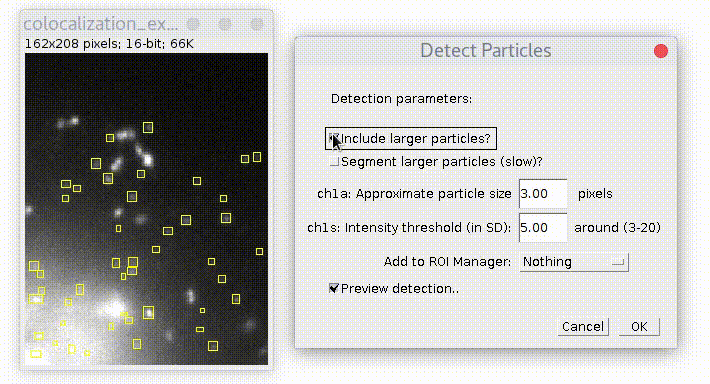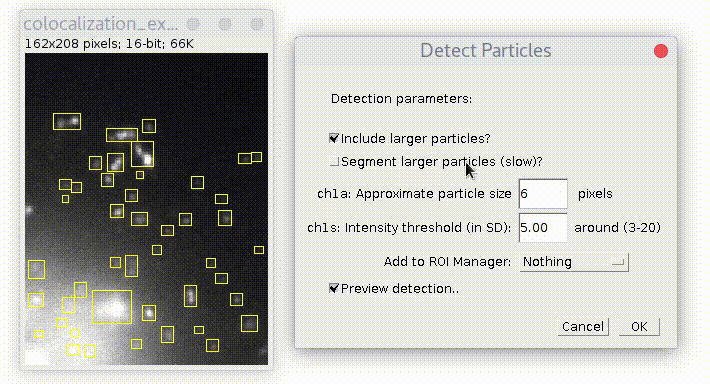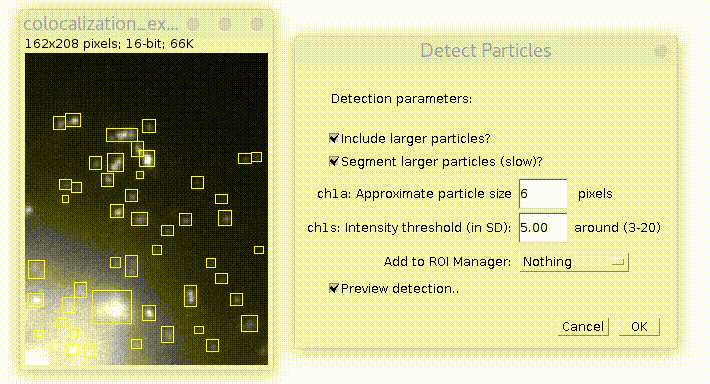
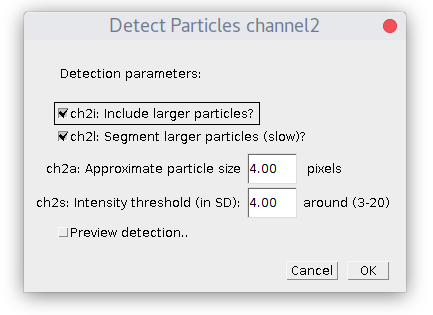
Specify estimated particles size and intensity threshold (particle brightness) and press OK. To have good detection usually you need to play with parameters and see how it goes. Checkbox "Preview..." allows to see detection on the current picture and simplifies this task. The "ROI shape" option allows you to choose, if you want your detection to be displayed as "Ovals" or "Rectangles".
By default plugin looks only for particles of specified size. If you check "Include larger particles?" box, it will also try to quantify bigger spots.
If "Segment larger particles (slow)?" box is checked, plugin will try to further split large particles into a smaller dots, if it is possible. As its name suggests, this procedure requires computational power and can be slow, especially on big images.
If you choose "Add to ROI Manager" option ("All particles"), plugin will add detected ROIs around particles to ROI Manager. ROIs will have names in the format of ind(detection# index in Results table)_ch(#channel)_sl(#slice)_fr(#frame)).
After pressing OK plugin runs and add ovals/rectangles in overlay on top of detected particles.
Also, it will provide you 'Results' table containing particles' coordinates (see below) and 'Summary' table. I recommend playing with parameters to get a nice detection result.
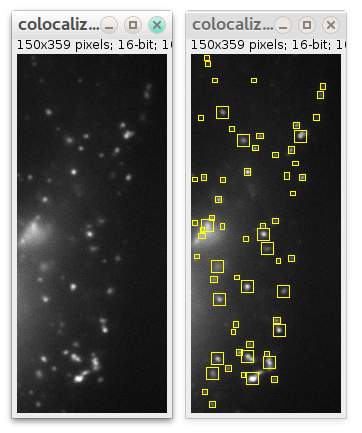
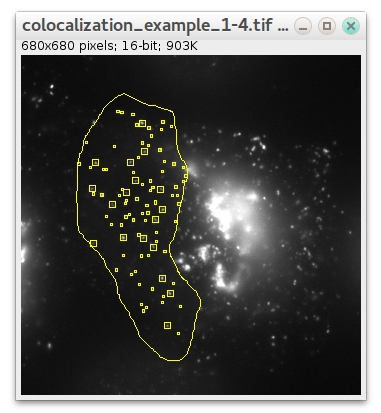
If you want detection to be performed in some specific region instead of whole image, select some ROI using any ImageJ ROI selection tools before launching plugin:
In this case only particles in that ROI will be detected (also supported in "Preview.." mode):
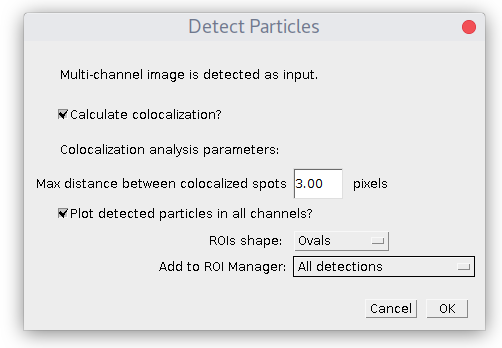
If your image contains multiple channels then after pressing 'Detect Particles' the dialog will look differently:
First, the plugin will show the window above with general setup and later it will proceed with a series of parameter windows, specifying detection parameters for each channel. To get colocalization analysis you need to check "Calculate colocalization?" box. If you uncheck it, then only detection will be performed.
There is an additional parameter in the case of colocalization: maximum distance between spots' centers. It defines at what maximum distance (in pixels) two spots in different channels are considered to be localized.
So colocalization is based on the distance between spots' centers!
After you press "OK", a series of windows for detection parameters for each channel will pop-up:
Take a notice that "Preview..." button in this case will only show detection, it will not mark/analyze colocalization.
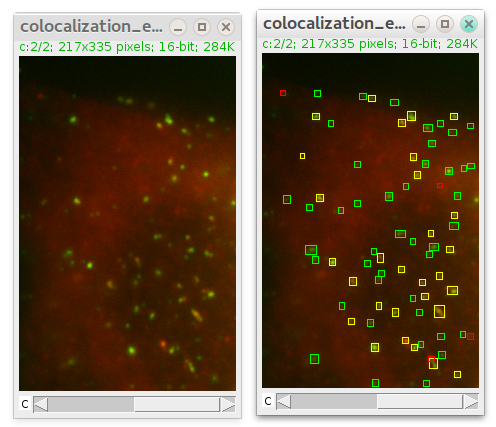
After detection is finished ComDet marks detected particles with ovals/rectangles of their own channel (LUT) color and colocalized particles in overlapping color:
Also "Add to ROI Manager" option is available. ROIs will have names in the format of ind(detection# index in Results table)_ch(#channel)_sl(#slice)_fr(#frame). You can add all detected particles or only those that colocalize.
Only ROI detection mode (as described above) also automatically works in this case.
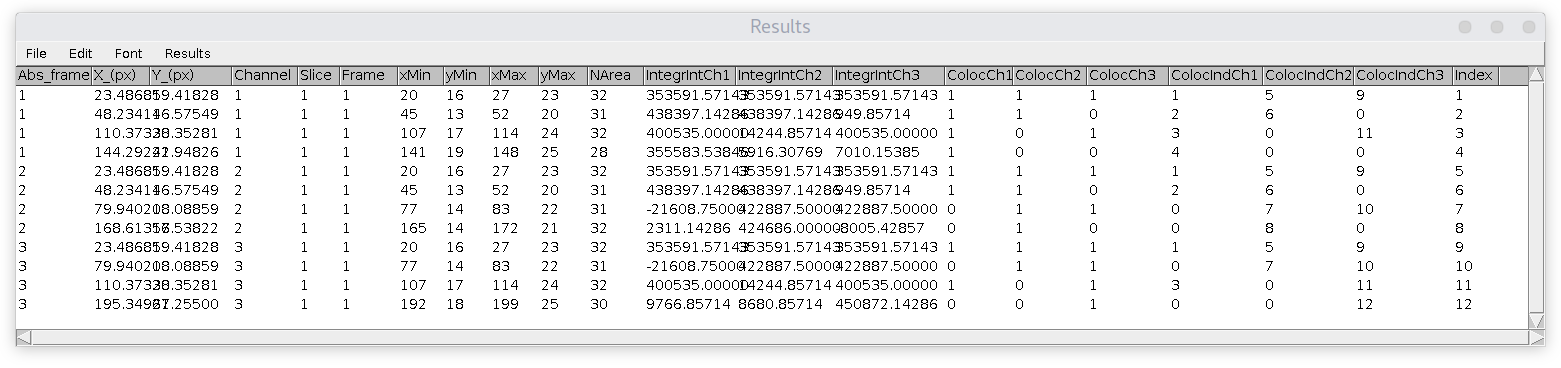
Here is an example of 'Results' table ('Summary' table is kind of self-explanatory).
The number of entries in this table is always equal to the number of detected particles in all channels, it is independent of colocalization analysis parameters.
Columns are:
-
Abs_frame = absolute frame number in stack/hyperstack (unique frame number)
-
X_(px) = x coordinate of spot (centroid fitting)
-
Y_(px) = y coordinate of spot (centroid fitting)
-
Channel = corresponding channel number, where the particle was detected
-
Slice = corresponding slice number (if z-stack)
-
Frame = corresponding frame number (if timelapse)
-
xMin, yMin, xMax, yMax = coordinate of rectangle around spot (in pixels)
-
NArea = the thresholded area of the spot in pixels
-
IntegrIntChX = for each channel (marked by X number) shows the spot's integrated intensity: the sum of all pixels intensity inside the thresholded area minus average spot-specific backgrounds. Background value is calculated as the average intensity of pixels along the perimeter of the rectangle. Notice that in case of colocalization, this value is calculated using the area/size of the spot obtained in detected channel.
-
ColocChX = equals to 1, if the spot is colocalized in this channel (marked by X number) and 0 otherwise. Notice, that it is always 1 in the channel, where the particle itself is detected (so it kind of colocalizes with itself). Also keep in mind, that if three particles from three different channels are colocalized, there would be three rows in the Results table. But! For three (and more) colors combinations there could be a situation when this value is less. For example, if particle in ch1 colocalized with ch2, ch2 colocalized with ch3, but ch1 and ch3 distance is larger the colocalization distance, so they are not colocalized. The main purpose of these columns is to provide you with information on this situation so you can perform a detailed analysis and extract the required info yourself (by filtering data in Excel or any other program).
-
ColocIndChX = corresponds to the unique detection index (last column) in Results table, corresponding to the particle in another channel (if colocalized). Zero otherwise.
-
Index = unique index for each detection.
Developed in Cell Biology group of Utrecht University.
Check out Updates history.
E-mail for any questions.