New issue
Have a question about this project? Sign up for a free GitHub account to open an issue and contact its maintainers and the community.
By clicking “Sign up for GitHub”, you agree to our terms of service and privacy statement. We’ll occasionally send you account related emails.
Already on GitHub? Sign in to your account
How to define the parameter 'Solubility gain per charge' #381
Comments
|
Dare @wangwei1619, the parameter "Solubility gain per charge" is only of relevance if you have a charged molecule. Kind regards, |
|
Dear @HenrikCordes Thanks for your answer. May I judge your mean as this parameter is for some molecules whose active form is being charged? Could you please give me some example of this kind of drug, because I am not familiar with this field? Best regards, |
|
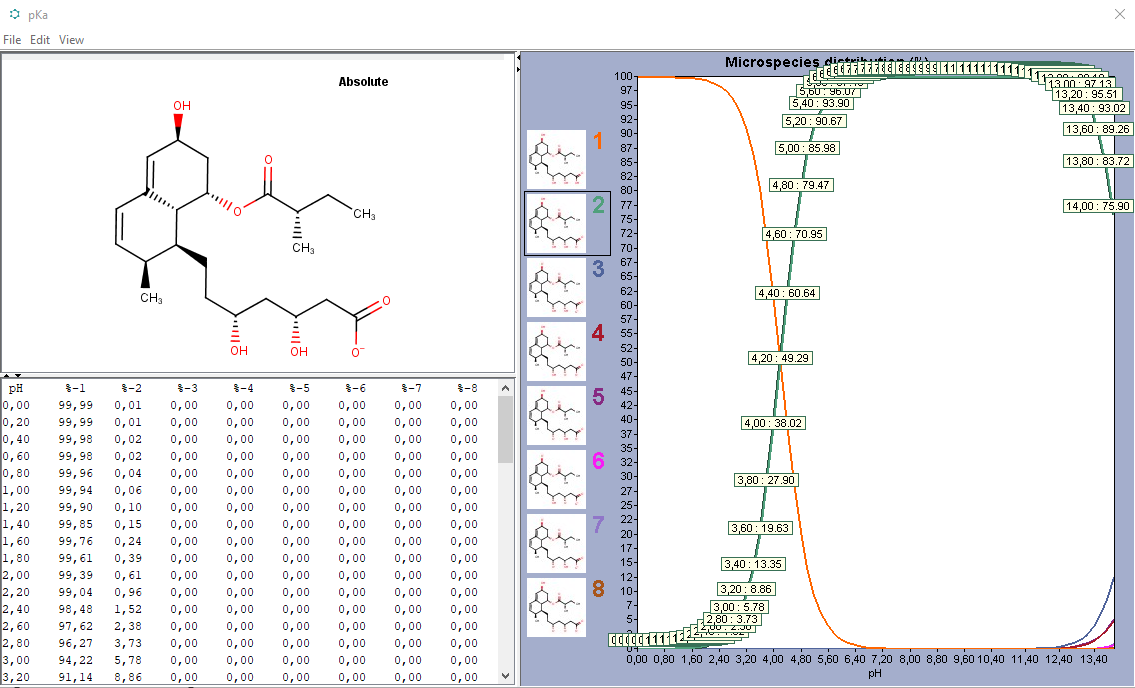
Dear @wangwei1619, it does not matter if a compound is active or not. Its a physico-chemical property of any compound. The molecular structure of the compound contains an acid group (bottom right of the molecules) with an pKa value of about 4. The acid function of the molecule can be of charge 0 or -1, depending on the environmental pH. Usually, the solubility of charged molecules is higher in an aqueous environment than for uncharged molecules and the factor "Solubility gain per charge" accounts for that behavior. Kind regards, |
|
Dear @HenrikCordes Thanks for your patience and specific description. However, as the molecule solubility and envrionment pH comform to this relationship: Best regards, |
|
Closing. Please reopen if you need more help |
Hi, all
Since I don't need to define any parameter related the ionization step of a molecule, what is this parameter Solubility gain per charge used for? Is it OK to always keep the default value of it?
The text was updated successfully, but these errors were encountered: