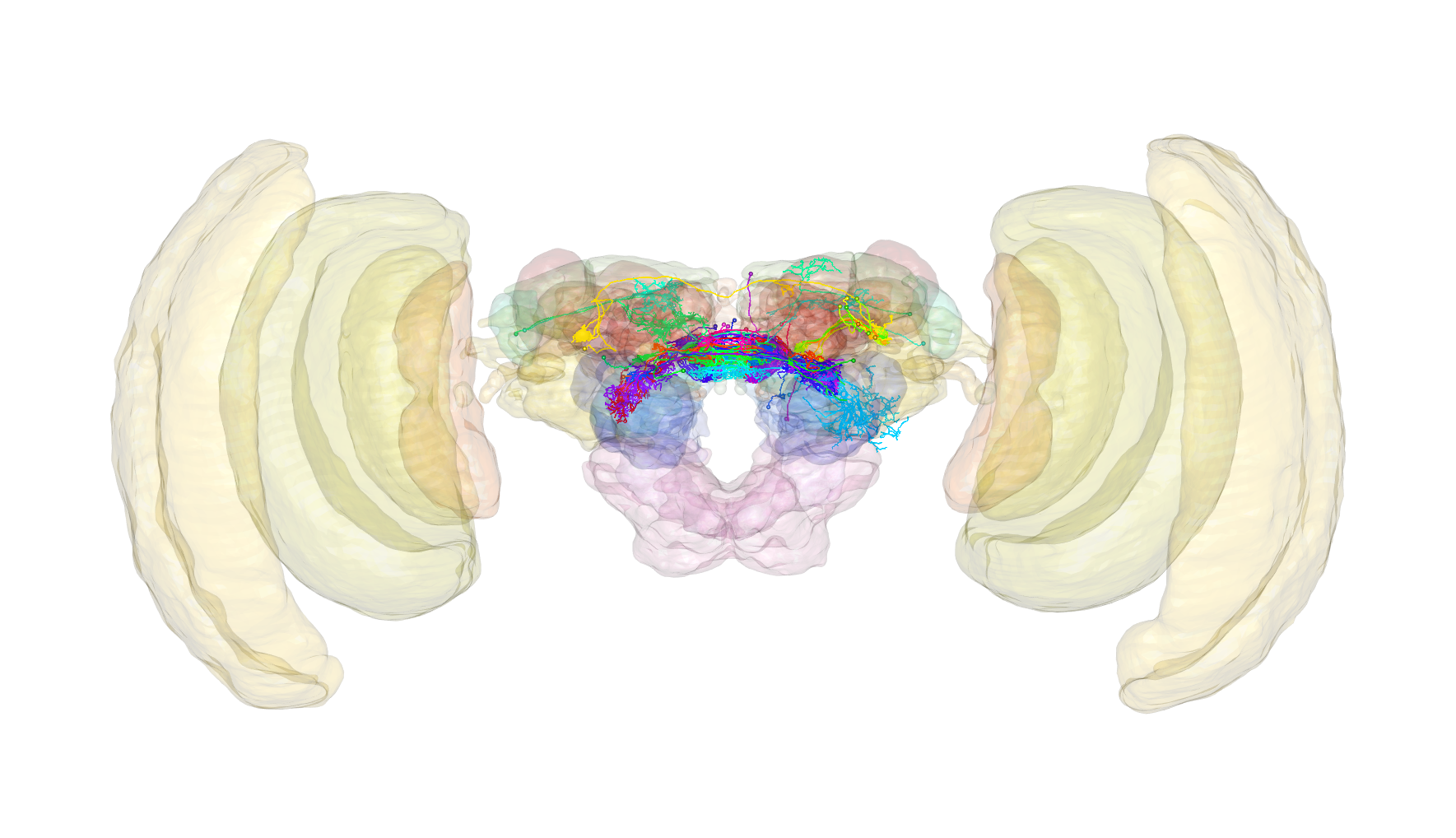
The goal of insectbrainr is to provide R client utilities for interacting with the Insect Brain Database. Using this R package in concert with the natverse ecosystem of neuroanatomy tools is highly recommended. The InsectBrainDB.org is primarily curated by Stanley Heinze. Learn more about the project here.
Firstly, you will need R, R Studio and X Quartz as well as nat and its dependencies. For detailed installation instructions for all this, see here. It should not take too long at all. Then:
# install
if (!require("remotes")) install.packages("remotes")
remotes::install_github("natverse/insectbrainr")
# use
library(insectbrainr)Done!
Now we can have a look at what is available, here are some of the key
functions. Their help details examples of their use. You can summon the
help in RStudio using ? followed by the function name.
# And how can I read neurons from the insectbrainDB?
?insectbrainr_read_neurons()
# Get 3D neuropil-subdivided brain models for those brainspaces
?insectbraindb_read_brain # Get 3D neuropil-subdivided brain models for those brainspacesLet’s also have a look at an example pulling neurons and brain meshes from insectbraindb.org. Here we shall take a look at neurons from the brain of the Monarch butterfly that have been registered to a template brain. Excitingly, we can also visualise this template brain.
## What neurons does the insectbraindb.org host?
available.neurons = insectbraindb_neuron_info()
## Let's just download all of the neurons in the database to play with,
## there are not very many:
nrow(available.neurons)
## First, we call the read neurons function, with ids set to NULL
insect.neurons = insectbraindb_read_neurons(ids = NULL)
## Hmm, let's see how many neurons we have perspecies
table(insect.neurons[,"common_name"])
## So, it seem the Monarch Butterfly is the clear winner there,
## maybe let's just have those
butterfly.neurons = subset(insect.neurons, common_name == "Monarch Butterfly")
## And let's plot them
nat::nopen3d(userMatrix = structure(c(0.999986588954926, -0.00360279157757759,
-0.00371213257312775, 0, -0.00464127957820892, -0.941770493984222,
-0.336223870515823, 0, -0.00228461623191833, 0.336236596107483,
-0.941774606704712, 0, 0, 0, 0, 1), .Dim = c(4L, 4L)), zoom = 0.600000023841858,
windowRect = c(1460L, 65L, 3229L, 1083L))
plot3d(butterfly.neurons, lwd = 2, soma = 5)
## Cool! But maybe we also want to see it's template brain?
## Let's check if they have it
available.brains = insectbraindb_species_info()
available.brains
## Great, they do, let's get it
butterfly.brain = insectbraindb_read_brain(species = "Danaus plexippus")
## And plot in a translucent manner
plot3d(butterfly.brain, alpha = 0.1)
## Oop, that's a lot of neuropils.
## Let's go for only a subset. What's available?
butterfly.brain$RegionList
butterfly.brain$neuropil_full_names
## There lateral horn (LH) and the antennal lobe (AL) are my favourites.
## Let's plot those
clear3d()
plot3d(subset(butterfly.brain, "LH|AL"), alpha = 0.5)
plot3d(butterfly.neurons, lwd = 2, soma = 5)
### Ffff, doesn't look like we have any neurons in my favourite neuropils :(The insectbraindb.org has a terms of use, which provides guidance on how best to credit data from these repositories. Most neurons have an associated publication that you can find on the repository websites.
This package was created by Alexander Shakeel Bates, while in the group of Dr. Gregory Jefferis. You can cite this package as:
citation(package = "insectbrainr")Bates AS (2019). insectbrainr: R client utilities for interacting with the InsectBrainDB.org. R package version 0.1.0. https://github.com/natverse/insectbrainr
The insectbraindb.org is primarily curated by Dr. Stanley Heinze, and was built by Kevin Tedore, and has several significant supporters, including the ERC.