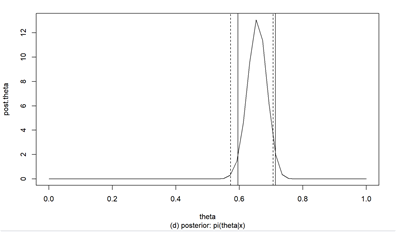
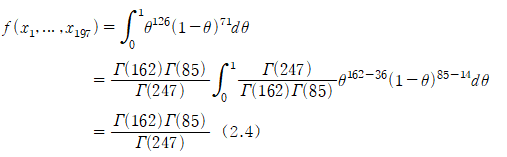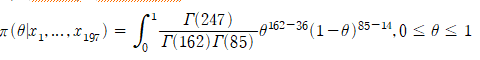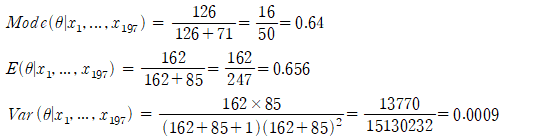Obtain the HPD post-distribution of Bayesian inference using prior information and confirm the importance of prior information and Bayesian inference compared to general confidence intervals
Data from 2011 to 2020 were used for the measurement period using data from phase 3 clinical trials of new drugs related to infectious diseases in the United States. In addition, data published in 2016 to use appropriate prior information (Biomedtracker. (2015). Clinical Development Success Rates 11, 2006-2015) will be checked and adopted as prior information, analyzed Bayesian inference, and predicted future clinical trial results.